Dr James Wheatley*, Dr Douglas Barnes** and Dr Helen Atkinson*** discuss a laboratory-based evaluation of the lifetime and degradation of C-Capture’s carbon capture system to assess the feasibility of implementation in the glassmaking sector.
Carbon capture technology for both industry and power generation is widely accepted as being essential in order for the UK government’s net zero target to be met.
Current carbon capture solvent technologies based on amines are energy intensive, and are susceptible to such extreme levels of solvent degradation under the conditions associated with glass manufacture as to be prohibitively expensive.
C-Capture has a solution: a more robust family of solvents, resistant to degradation, which require less energy to use and are more environmentally benign than existing technologies.
Recent work at C-Capture has begun to demonstrate its proprietary solvent technology to be more robust to conditions including high levels of SOx, NOx and O2. Here it presents some initial data from these experiments, which demonstrate how C-Capture’s technology may provide sectors which have traditionally been deemed challenging to decarbonise a credible solution.

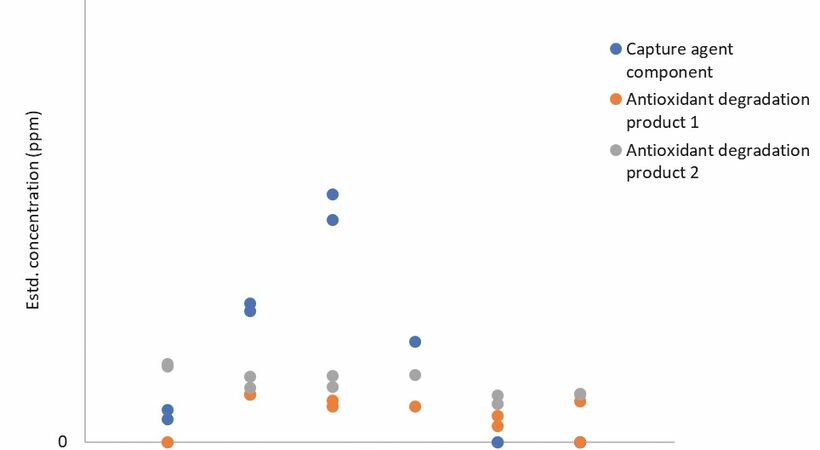
Fig 2 .Impurities observed under ~2000ppm oxygen.
Introduction
C‑Capture designs chemical processes for the capture of carbon dioxide (CO2). It has patented a safe, low‑cost post‑combustion capture technology which uses up to 40% less energy than current commercially available technologies.
Carbon capture offers a crucial decarbonisation route for industries such as glassmaking and cement manufacture, for which carbon emissions are inherent to their processes and cannot be addressed by other means such as energy efficiency improvements, or fuel switching.
In order for these industries to decarbonise, they must have access to a carbon capture technology which is both effective and economical. The Committee on Climate Change recently stated ‘Business models that are not compatible with a Net Zero future are increasingly risky’. If carbon capture technologies cannot meet industry requirements, it is possible to imagine a future in which foundation industries such as glass manufacturing become incompatible with a low‑carbon UK economy, and are forced to scale back their operations or even move offshore.
Carbon capture on glass manufacture is challenging, due to the high levels of impurities found in the flue gas, particularly SOx and NOx. The current state‑of‑the‑art for carbon capture are amine‑based solvents which were initially developed in the 1930s. Whilst undoubtedly an effective technology in many respects, amine‑based solvents have limitations including:
- Energy intensive regeneration
- Susceptibility to degradation (especially under harsh conditions)
- High levels of corrosivity
- Potential human or environmental toxicity
- Potential carcinogenicity either of the components themselves or their degradation products.
Taken together, these factors have deterred widespread adoption, particularly in challenging applications such as glass manufacturing. C‑Capture’s proprietary solvents are entirely amine, and nitrogen, free, being based on a fundamentally different underlying chemistry which offers numerous benefits including reduced energy input, high stability under all conditions evaluated thus far, low corrosivity, and minimal production of hazardous by‑products.
In these experiments, we are expanding our dataset and knowledge of solvent performance under challenging conditions, including high levels of SOx and NOx, alongside comparable levels of O2 and thermal degradation.
Experimental
Four solvent ageing systems of novel design, tailor-made for the analysis of C-Capture’s capture solvent, were designed, built and commissioned. Modifications to C-Capture’s in-house GC-MS and GC-FID analysis systems were made to allow analysis of solvent degradation species.
Solvent ageing experiments were carried out under condition of:
- 140°C, nitrogen atmosphere.
- 140°C, nitrogen/air (balance ~2000 ppm oxygen).
- 140°C, nitrogen/sulphur dioxide (2000 ppm).
- 140°C, nitrogen/nitric oxide (2000 ppm).
Experiments were carried out over a two month period. Suspected degradation products were analysed by GC-FID and GC-MS to provide a comparison across the different sets of conditions.

Fig 3. Impurities observed under 2000ppm SO2.
Results
Thermal degradation C-Capture’s proprietary CO2 capture solvent was aged at 140°C under a pressurised nitrogen atmosphere (5 bar). As can be seen in figure 1, minimal (<70 ppm total) levels of suspected degradation products were detected by GC-MS throughout the experimental period, suggesting overall solvent stability is high under these conditions. Of these products, three were identified: a component of the capture agent; and two degradation products of the antioxidant included in the solvent (>95% of the antioxidant remained in its initial form). No clear trend in concentration was found, and therefore there is insufficient evidence at present to characterise any degradation rate.
Degradation in the presence of O2 C-Capture’s solvent was in the presence of ca.2000 ppm oxygen. As can be seen in fig. 2, a slightly higher level of suspected degradation products was initially observed however this declined to only ~10ppm by the end of the experiment. Again, the overall level of observed degradation was very low and the evidence is that the solvent appears highly robust under these conditions.
Degradation in the presence of SO2 C-Capture’s solvent was aged in the presence of 2000ppm SO2, mirroring the high levels of sulfur oxides found in glassmaking and other heavy-industry flue gases. As can be seen in fig. 3, very low levels of suspected degradation products were observed, falling down to below the limit of detection (~1 ppm) by GC at the end of the experiment.
Degradation in the presence of NO C-Capture’s solvent was aged in the presence of 2000ppm NO, imitating the high levels of nitrogen oxides also found in glassmaking flue gases. NO constitutes the major nitrogen oxide found in most flue gases, subsequently equilibrating to produce NO2 only in the atmosphere, and therefore was prioritised for this work.
As can be seen in fig. 4, levels of suspected degradation products were once again very low, providing further support to C-Capture’s claims regarding the robustness of its solvent under harsh conditions.

Fig 4. Impurities observed under 2000ppm NO.
Conclusion
Though these results are only preliminary, they are extremely promising. C-Capture has successfully demonstrated the feasibility of deploying its technology in challenging conditions, such as may be presented by glass manufacture.
The resistance to oxidation and the robust nature of C-Capture’s solvent will be welcome news to industrial emitters where operating conditions are particularly harsh but decarbonisation is still urgently needed.
Carried out with support from Innovate UK via the Sustainable Innovation Fund.
*Senior Chemist, **Head of Chemistry, ***Business Development Manager, C-Capture, Leeds, UK,
www.c-capture.co.uk/
*h.atkinson@c-capture.co.uk